Electrochemical Series: Tips and Tricks to learn
Learn the simple tips and tricks for Electrochemical Series. Also, learn the tricks for the reactivity series

The arrangement of elements in order of increasing electrode potential value is called electrochemical series. Electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potential. The most negative E0 value is placed at the top of the electrochemical series and most positive at the bottom:
Trick to Learn Electrochemical Series
Likh K Bara Sardar Ka Nam Maggie Ali Jakar Feko Cd Koi Nyi(Ni) jis cd me Sunder Pyara Bappi jo H Cute phn k rakhta hai chandi (Ag) or sona (Au) forever
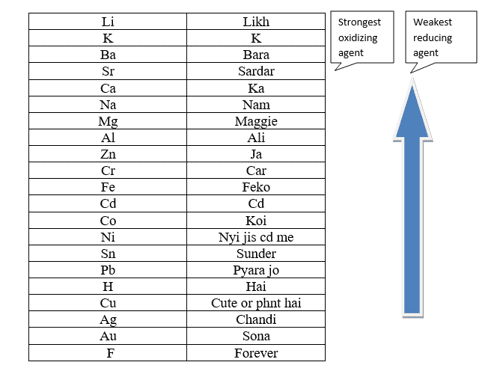
Advantages of Electrochemical Series
1. Comparison of relative oxidizing and reducing power of various metals
A Positive number indicates a stronger reduction potential of the electrode and it functions as a stronger Oxidizing agent. A Negative number means weaker reduction potential of the electrode, and hence it is a more powerful Reducing agent.
2. For comparison of the relative activities of metals towards displacement reactions based on their placement in activity series
As all the metal displacement reactions are cation displacement reactions the rule that “ The element that forms cation can displace only cation and elements that form the anion can displace any anion” is applicable.
Greater the oxidation potential of metal, more quickly can it undergo loss of electron and greater is its reactivity. It means a metal that is higher inactivity series can displace other metal that is placed lower in reactivity series.
- REACTIVITY SERIES IS
K > Na > Ca > Mg > Al > Zn > Fe > Ni > Sn > Pb > H > Cu > Hg > Ag > Au > Pt
TRICK TO LEARN THE REACTIVITY SERIES
i)Remember the following sentence :
Kedar Nath Ka Mali Aloo Zara Feke Nhi Pkata Hai
- K – KEDAR
- Na – NATH
- Ca – KA
- Mg – MALI
- Al – ALOO
- Zn – Zara
- Fe – FEKE
- Ni – Nhi
- Pb – PKATA
- H - HAI
- ii) Keep in mind the next word i.e CHAAP which represent
C- COPPER , H- HYDROGEN , Ag – SILVER, Au – GOLD, Pt – Platinum
Use of reactivity series:
If metals Zn and Ag are placed in an aqueous solution of CuSO4 , The blue colour solution changes to colourless (or fades) in which set.
Zn is more reactive than Cu and thus Zn reacts with CuSO4 and form ZnSO4 and Cu and solution changes to colourless
If Ag is placed in CuSO4 Ag is less reactive than Cu hence cannot replace Cu hence no reaction take place and there is no change in colour of solution.
3. CHECK WETHER GIVEN METAL IS MORE REACTIVE OR LESS REACTIVE THAN HYDROGEN
As we see in electrochemical series the HYDROGEN is placed between copper and lead. The metals on the left side of Hydrogen are generally more reactive and on right side are less reactive
NOTE:
The metal more reactive than hydrogen can replace the hydrogen and on the other hand the metals less reactive than hydrogen can’t eliminate the hydrogen. We can say metals of high reactivity(metals on left side of hydrogen) can replace the metals of less reactivity(metals on the right side of the hydrogen.
4. To predict weather a metal reacts with acid to librate hydrogen gas
The metal undergo oxidation and the hydrogen ion by accepting the electron undergo reduction. If metal has negative reduction potential and is place above the hydrogen in electrochemical series it can react with an acid to librate hydrogen gas.
Chemical reaction looks like:
M(S) + H+(aq) -à M+(aq) + ½ H2(g)
5. To calculate the standard EMF of any electrochemical cell (E0cell)
The potential difference between anode and cathode in a cell is called electromotive force or EMF The standard EMF of the cell is an addition product of standard electrode potential of two half cells i.e reduction half – cell and oxidation half – cell
E0cell = E0reduction + E0oxidation
Standard oxidation potential = - standadtd reduction potential
As anode is the negative terminal for oxidation and cathode for reduction hence
E0cell = E0cathode + E0anode
Some Important Points to Always Keep in Mind
- In electrochemical series electropositivity decrease from top to bottom, the thermal stability of oxide also decreases from top to bottom
- The oxides of metals having high positive reduction potentials are not stable towards heat.
- The metals which come below copper form unstable oxides, i.e., these are decomposed on heating.
- Reducing nature depends on the tendency of losing electron or electrons. More the negative reduction potential more is the tendency to lose electron or electrons.
- Thus, reducing nature decreases from top to bottom in the electrochemical series.
- A metal higher in the series will displace the metal from its solution which is lower in the series, i.e., the metal having low standard reduction potential will displace the metal from it's salt's solution which has higher value of standard reduction potential.
- Strongly electropositive metals: Metals having standard reduction potential near about -2.0 volt or more negative like alkali metals, alkaline earth metals are strongly electropositive in nature.
- Moderately electropositive metals: Metals having values of reduction potentials between 0.0 and about -2.0 volt are moderately electropositive. Al, Zn, Fe, Ni, Co, etc., belong to this group.
- Weakly electropositive metals: The metals which are below hydrogen and possess positive values of reduction potentials are weakly electropositive metals. Cu, Hg, Ag, etc., belong to this group.
- Alkali metals and alkaline earth metals having high negative values of standard reduction potentials are chemically active. These react with cold water and evolve hydrogen.
- The anion which is a stronger reducing agent (low value of standard reduction potential) is liberated first at the anode.
- A more electropositive metal can displace a less electropositive metal from its salt's solution. This principle is applied for the extraction of Ag and Au by the cyanide process.
JEE Main Syllabus with weightage
JEE Main Question Papers with Solutions
All the best!
Team Goprep
Tags

Mohit Chauhan
